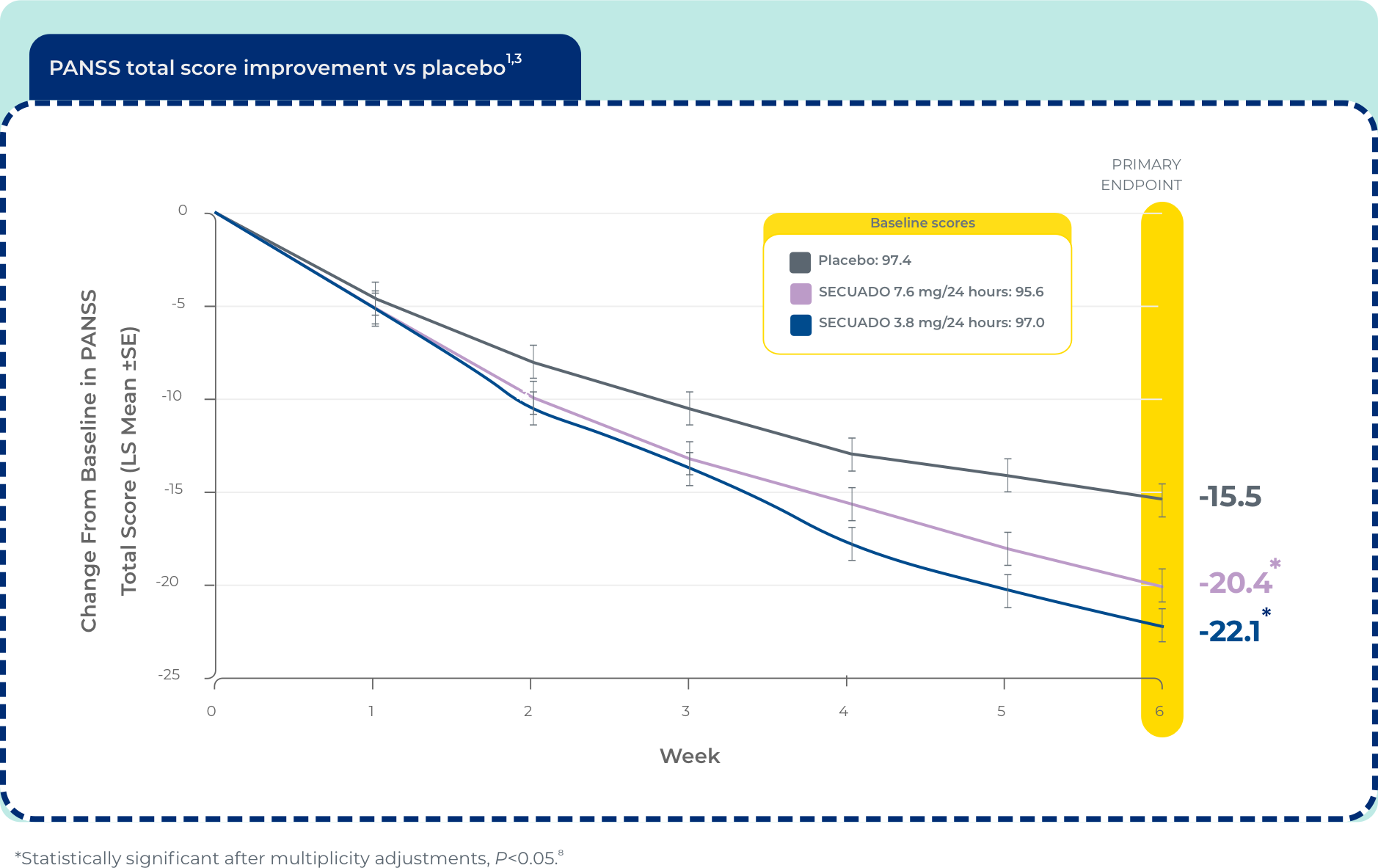
Significant reduction in PANSS total score at week 6 (n=607)1
- Both doses of SECUADO® were also statistically superior to placebo for Clinical Global Impressions – Severity (CGI-S)1
- An examination of population subgroups did not reveal any clear evidence of differential responsiveness on the basis of age, sex, and/or race1
The efficacy of SECUADO® was evaluated in a 6-week, fixed-dose, randomized, double-blind, placebo-controlled trial (Study 1; NCT 02876900) of adult patients who met DSM-IV® criteria for schizophrenia. The PANSS and CGI-S rating scales were used as the primary and key secondary efficacy measures, respectively, for assessing psychiatric signs and symptoms in each trial.1
PANSS is a 30-item scale that measures positive symptoms of schizophrenia (7 items), negative symptoms of schizophrenia (7 items), and general psychopathology (16 items), each rated on a scale of 1 (absent) to 7 (extreme); total PANSS scores range from 30 to 210.
CGI-S is a validated clinician-related scale that measures the patient’s current illness state and overall clinical state on a 1-point (normal, not at all ill) to 7-point (extremely ill) scale, based on the rater’s total clinical experience with this population.1
DSM-IV® = Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition); LS = least squares; PANSS = Positive and Negative Syndrome Scale; SE = standard error.
DSM-IV® is a registered trademark of the American Psychiatric Association.