Other clinical data
Sublingual asenapine
• The efficacy of SECUADO® was established, in part, on data from trials with the sublingual formulation of asenapine1
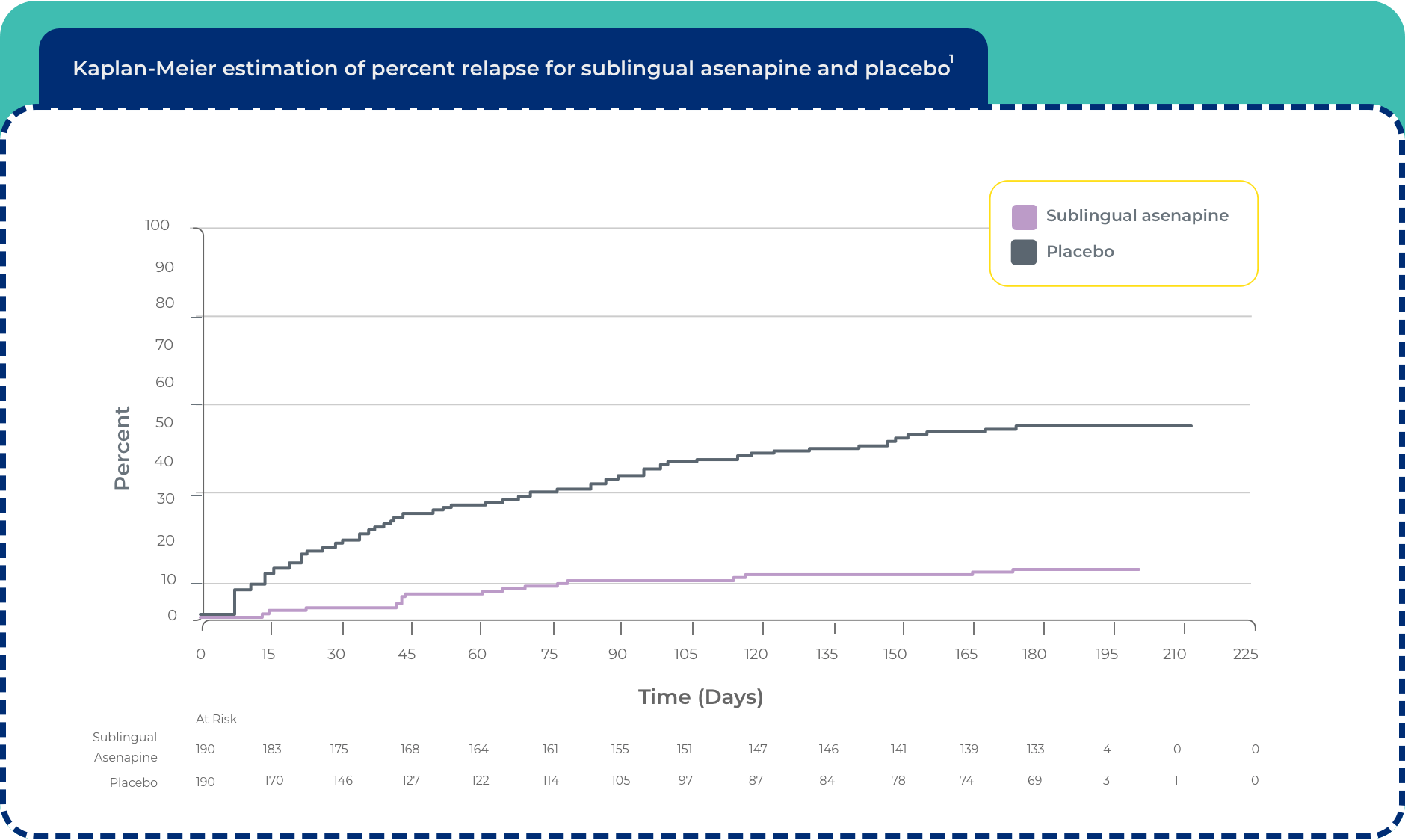
Maintenance of efficacy with sublingual asenapine1
Maintenance of efficacy has been demonstrated in a placebo-controlled, double-blind, multicenter, flexible dose with sublingual asenapine (5 mg or 10 mg twice daily based on tolerability) clinical trial with a randomized withdrawal design. All patients were initially administered 5 mg twice daily for 1 week and then titrated up to 10 mg twice daily. A total of 700 patients entered open-label treatment with sublingual asenapine for a period of 26 weeks. Of these, a total of 386 patients who met pre-specified criteria for continued stability (mean length of stabilization was 22 weeks) were randomized to a double-blind, placebo-controlled, randomized withdrawal phase. Sublingual asenapine was statistically superior to placebo in time to relapse or impending relapse defined as increase in PANSS ≥20% from baseline and a CGI-S score ≥4 (at least 2 days within 1 week) or PANSS score ≥5 on “hostility” or “uncooperativeness” items and CGI-S score ≥4 (≥2 days within a week), or PANSS score ≥5 on any 2 of the following items: “unusual thought content,” “conceptual disorganization,” or “hallucinatory behavior” items, and CGI-S score ≥4 (≥2 days within 1 week) or investigator judgment of worsening symptoms or increased risk of violence to self (including suicide) or other persons.