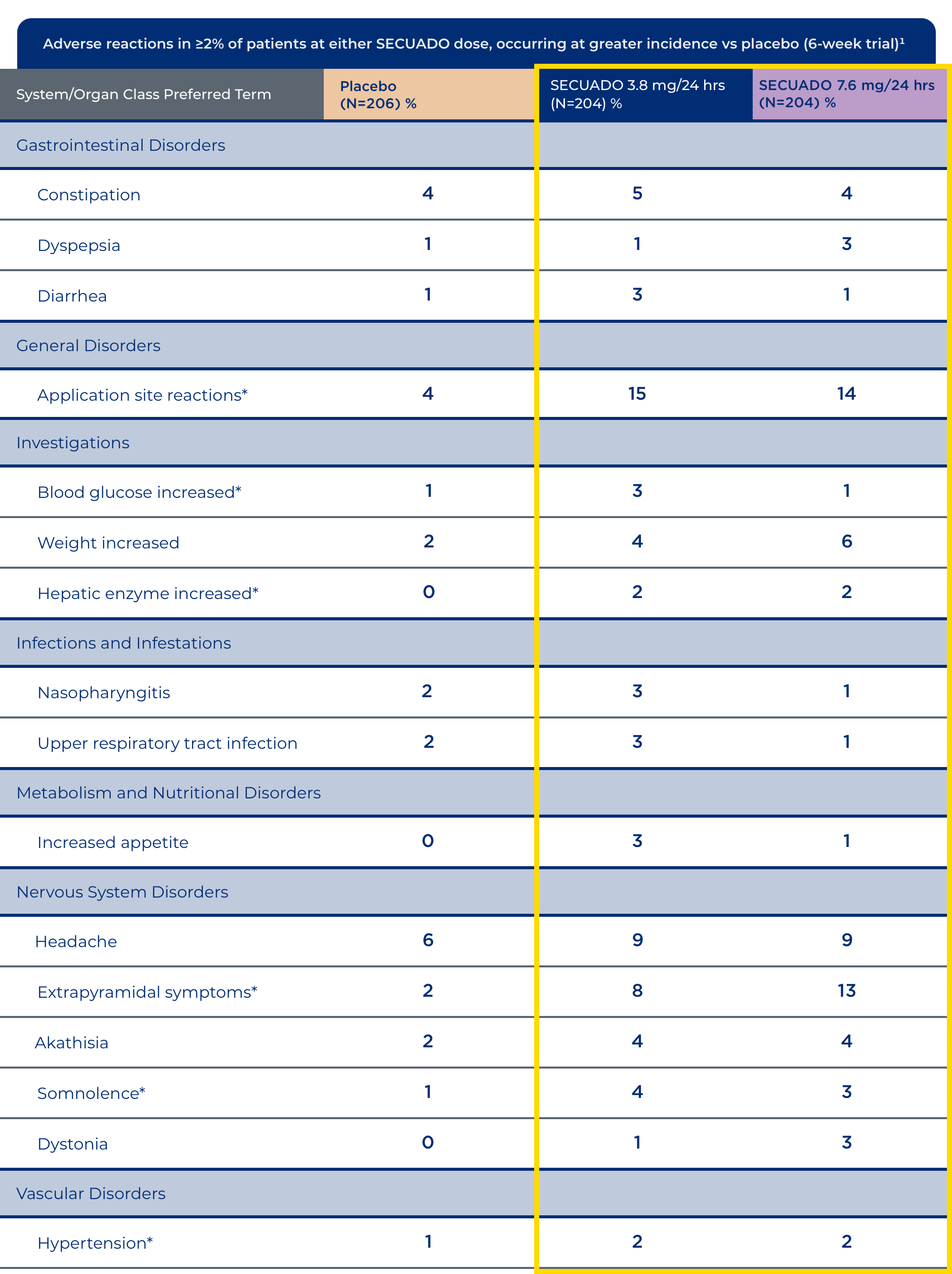
Established safety and tolerability: IT’S ON™
Commonly observed adverse reactions (incidence ≥5% and at least twice the rate of placebo) were extrapyramidal disorder, application site reaction, and weight gain1
SECUADO® had a discontinuation rate similar to placebo1
- A total of 4.9% of patients treated with SECUADO 3.8 mg/24 hours, 7.8% of patients treated with SECUADO 7.6 mg/24 hours, and 6.8% of patients on placebo discontinued due to adverse reactions
- The adverse reaction that most commonly led to discontinuation among patients treated with SECUADO in this trial was akathisia, which led to discontinuation in 0% (0/204) of patients treated with SECUADO 3.8 mg/24 hours, 1.5% (3/204) of patients treated with SECUADO 7.6 mg/24 hours, and 0.5% (1/206) of patients on placebo
*The following terms were combined: Application site reactions includes application site dermatitis, discoloration, discomfort, dryness, edema, erythema, exfoliation, induration, irritation, pain, papules, pruritus, and reaction. Blood glucose increased includes blood glucose increased, blood insulin increased, glycosylated hemoglobin increased, hyperglycemia, Type 2 diabetes mellitus, diabetes mellitus, and hyperinsulinemia. Hepatic enzyme increased includes hepatic enzyme increased, alanine aminotransferase increased, aspartate aminotransferase increased, and gamma-glutamyl transferase increased. Extrapyramidal symptoms includes dyskinesia, dystonia, extrapyramidal disorder, parkinsonism, tardive dyskinesia, muscle spasm, and musculoskeletal stiffness. Somnolence includes somnolence, sedation, lethargy, and hypersomnia. Hypertension includes hypertension, blood pressure increased, diastolic hypertension, and hypertensive crisis.1