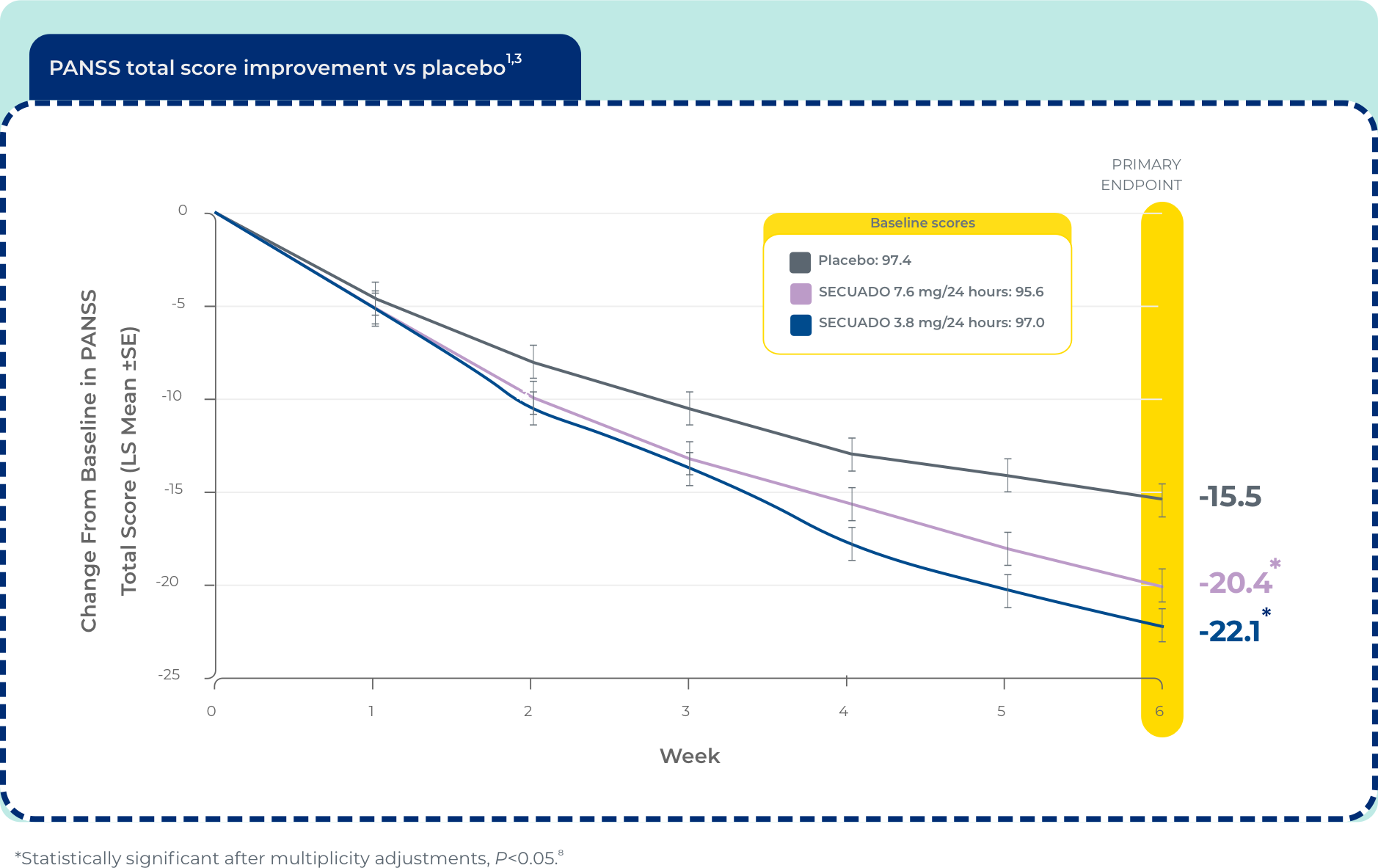
Significant reduction in PANSS total score at week 6 (n=607)1
- Both doses of SECUADO® were also statistically superior to placebo for Clinical Global Impressions – Severity (CGI-S)1
- An examination of population subgroups did not reveal any clear evidence of differential responsiveness on the basis of age, sex, and/or race1
The efficacy of SECUADO® was evaluated in a 6-week, fixed-dose, randomized, double-blind, placebo-controlled trial (Study 1; NCT 02876900) of adult patients who met DSM-IV® criteria for schizophrenia. The PANSS and CGI-S rating scales were used as the primary and key secondary efficacy measures, respectively, for assessing psychiatric signs and symptoms in each trial.1
PANSS is a 30-item scale that measures positive symptoms of schizophrenia (7 items), negative symptoms of schizophrenia (7 items), and general psychopathology (16 items), each rated on a scale of 1 (absent) to 7 (extreme); total PANSS scores range from 30 to 210.
CGI-S is a validated clinician-related scale that measures the patient’s current illness state and overall clinical state on a 1-point (normal, not at all ill) to 7-point (extremely ill) scale, based on the rater’s total clinical experience with this population.1
DSM-IV® = Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition); LS = least squares; PANSS = Positive and Negative Syndrome Scale; SE = standard error.
DSM-IV® is a registered trademark of the American Psychiatric Association.
Other clinical data
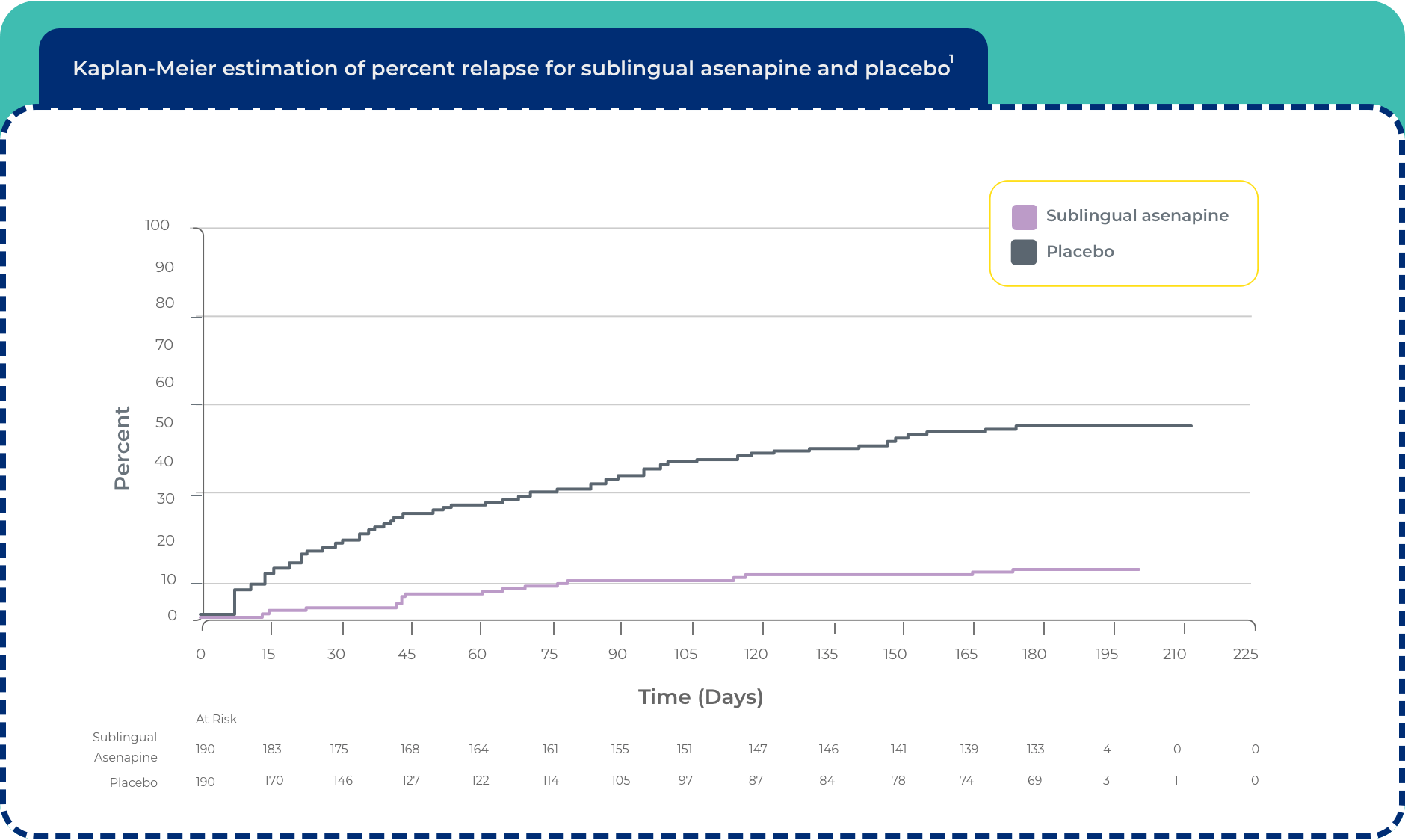
Sublingual asenapine
• The efficacy of SECUADO® was established, in part, on data from trials with the sublingual formulation of asenapine1
Maintenance of efficacy with sublingual asenapine1
Maintenance of efficacy has been demonstrated in a placebo-controlled, double-blind, multicenter, flexible dose with sublingual asenapine (5 mg or 10 mg twice daily based on tolerability) clinical trial with a randomized withdrawal design. All patients were initially administered 5 mg twice daily for 1 week and then titrated up to 10 mg twice daily. A total of 700 patients entered open-label treatment with sublingual asenapine for a period of 26 weeks. Of these, a total of 386 patients who met pre-specified criteria for continued stability (mean length of stabilization was 22 weeks) were randomized to a double-blind, placebo-controlled, randomized withdrawal phase. Sublingual asenapine was statistically superior to placebo in time to relapse or impending relapse defined as increase in PANSS ≥20% from baseline and a CGI-S score ≥4 (at least 2 days within 1 week) or PANSS score ≥5 on “hostility” or “uncooperativeness” items and CGI-S score ≥4 (≥2 days within a week), or PANSS score ≥5 on any 2 of the following items: “unusual thought content,” “conceptual disorganization,” or “hallucinatory behavior” items, and CGI-S score ≥4 (≥2 days within 1 week) or investigator judgment of worsening symptoms or increased risk of violence to self (including suicide) or other persons.
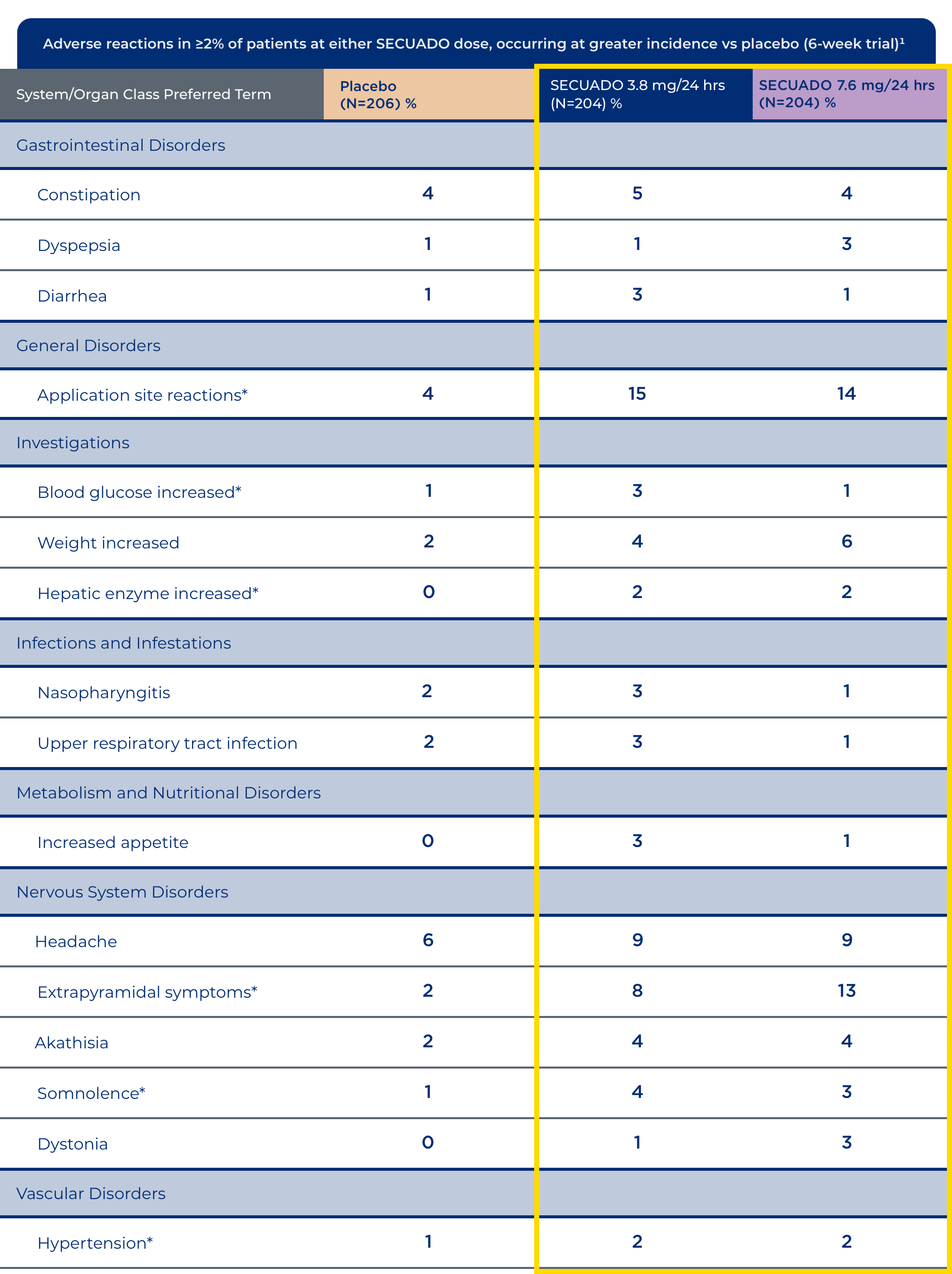
Established safety and tolerability: IT’S ON™
Commonly observed adverse reactions (incidence ≥5% and at least twice the rate of placebo) were extrapyramidal disorder, application site reaction, and weight gain1
SECUADO® had a discontinuation rate similar to placebo1
- A total of 4.9% of patients treated with SECUADO 3.8 mg/24 hours, 7.8% of patients treated with SECUADO 7.6 mg/24 hours, and 6.8% of patients on placebo discontinued due to adverse reactions
- The adverse reaction that most commonly led to discontinuation among patients treated with SECUADO in this trial was akathisia, which led to discontinuation in 0% (0/204) of patients treated with SECUADO 3.8 mg/24 hours, 1.5% (3/204) of patients treated with SECUADO 7.6 mg/24 hours, and 0.5% (1/206) of patients on placebo
*The following terms were combined: Application site reactions includes application site dermatitis, discoloration, discomfort, dryness, edema, erythema, exfoliation, induration, irritation, pain, papules, pruritus, and reaction. Blood glucose increased includes blood glucose increased, blood insulin increased, glycosylated hemoglobin increased, hyperglycemia, Type 2 diabetes mellitus, diabetes mellitus, and hyperinsulinemia. Hepatic enzyme increased includes hepatic enzyme increased, alanine aminotransferase increased, aspartate aminotransferase increased, and gamma-glutamyl transferase increased. Extrapyramidal symptoms includes dyskinesia, dystonia, extrapyramidal disorder, parkinsonism, tardive dyskinesia, muscle spasm, and musculoskeletal stiffness. Somnolence includes somnolence, sedation, lethargy, and hypersomnia. Hypertension includes hypertension, blood pressure increased, diastolic hypertension, and hypertensive crisis.1